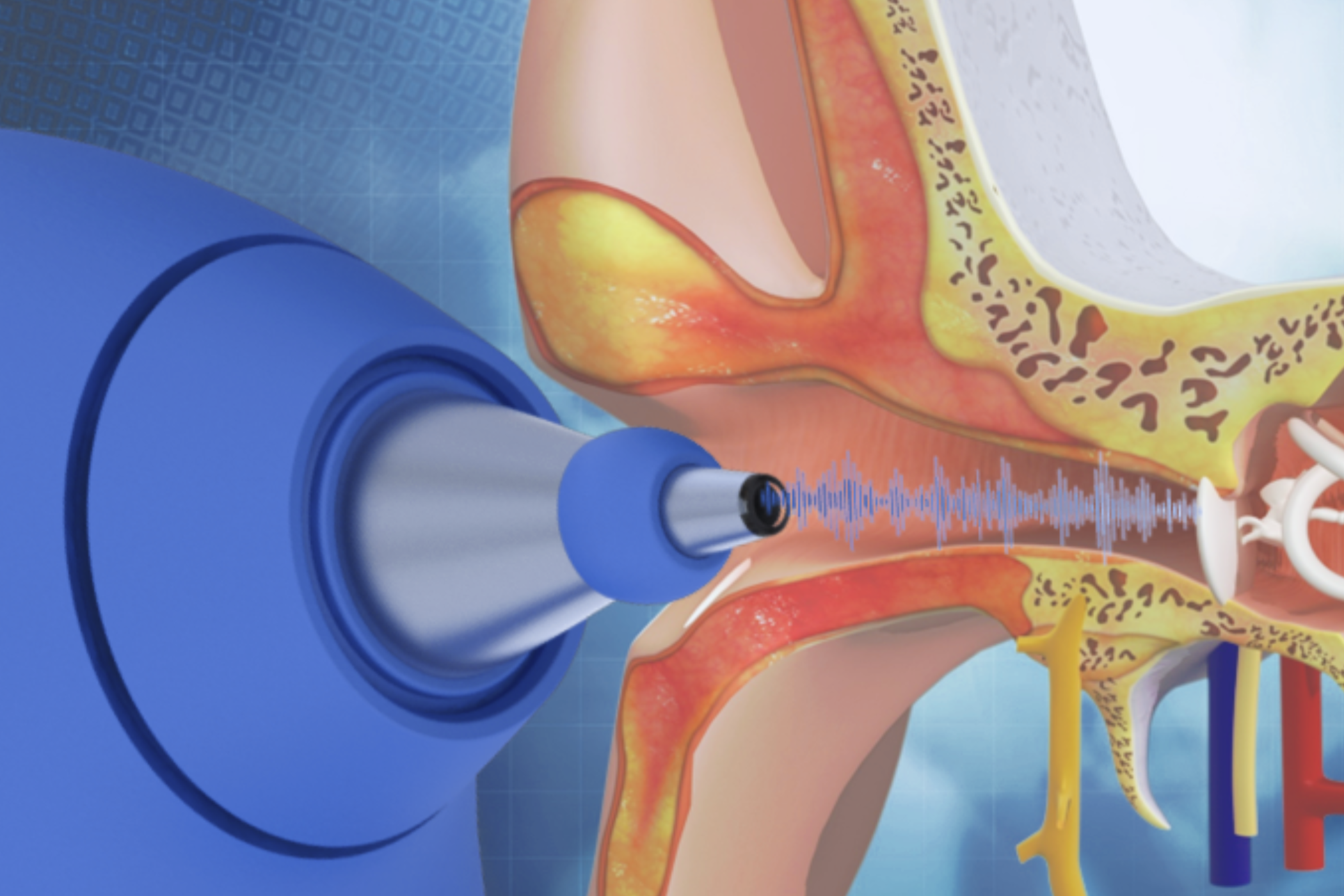
Objective middle-ear infection evaluation at the point of care.
OtoNexus Medical Technologies is developing an ultrasound otoscope designed to reduce diagnostic uncertainty, support antibiotic stewardship, and lower system costs.
Key Highlights
Selected for U.S. FDA Safer Technologies Program (STeP) Learn more.
2-second scan workflow for frontline settings
Razor/razorblade economics: Device + single-use tips.
Early clinical work at leading institutions supports ongoing development.
The OtoNexus Solution
Unmet Need:
Otitis Media is common; treatment accuracy is inconsistent.
Clinical Value:
Objective data at the visit support better decision-making.
System Value:
Potential reduced unnecessary antibiotics and downstream costs.
-
OtoNexus has been selected for the U.S. Food and Drug Administration (FDA) Safer Technologies Program (STeP), which is intended to facilitate development and review for certain devices that may offer meaningful safety advantages over existing alternatives. Participation in STeP supports earlier and more structured engagement with FDA to align on evidence expectations, development priorities, and submission readiness. This can improve execution predictability by helping teams address key regulatory questions earlier in the development cycle. STeP selection is a program designation and does not represent FDA clearance or approval.
-
OtoNexus’s commercial model combines capital device placement with recurring consumables revenue. The ultrasound otoscope is sold (or placed) as durable equipment, creating an installed base in clinics. Each exam uses a single-use disposable tip, which supports infection control and generates repeat purchase volume tied directly to utilization. As adoption grows and exam volume increases, consumable demand scales with the installed base—creating recurring revenue alongside device sales.
-
The market opportunity for the OtoNexus ultrasound otoscope is huge, with a multi-billion-dollar yearly revenue potential in the U.S. alone and even greater opportunities globally. This is a blue ocean market space with immense growth potential, as no other product on the market can identify bacterial middle ear infections with the precision and speed that our device offers. The ability to accurately detect bacterial infections provides the critical information physicians need to determine the appropriateness of antibiotic therapy—a capability that positions OtoNexus as a leader in reducing unnecessary antibiotic prescriptions.
-
Beyond its current application in detecting middle ear infections, the underlying ultrasound technology platform has additional potential for both medical and industrial uses, all of which are protected by patents. This opens up further avenues for growth and revenue diversification. OtoNexus has received over 76 patents for this unique technology, creating a strong patent moat that strengthens the company’s competitive edge, protects its technology and revenue streams, and enhances market leadership.tem description
Key Highlights and Benefits:
Accuracy and clinical insight
Designed to detect the presence of middle ear effusion and help characterize infection type to support appropriate management.
Speed and efficiency at the point of care
Designed to return results in seconds through a simplified, one-button workflow—reducing exam time and supporting timely decision-making during the visit.
Antibiotic stewardship
Intended to help clinicians distinguish cases where antibiotics may be appropriate versus cases more consistent with non-bacterial presentations—reducing avoidable prescribing and improving precision in antibiotic use.
Learn more about our new technology here.
Product Development Achievements:
Patented Technology: Our proprietary application of through-air ultrasound, miniaturized to fit within the tip of an ear speculum, is patented, providing a competitive edge in the market.
Clinical Validation: We have conducted successful clinical studies demonstrating the ultrasound otoscope's high accuracy and reliability, validating its effectiveness.
Strategic Partnerships: We have established collaborations with leading healthcare institutions and organizations to further our mission and enhance our market reach.
Team & Governance
OtoNexus is led by an experienced team with backgrounds across medical device development, research and engineering, clinical and regulatory execution, and commercialization. The leadership team is focused on advancing the program through key development and market-readiness milestones and building the organizational capabilities required to support adoption and scale. Learn more about our team here.
Company Focus
OtoNexus is developing a point-of-care approach intended to improve identification and management of middle ear infections by reducing diagnostic uncertainty in frontline settings.
Antibiotic resistance is a significant public health and economic issue, and avoidable antibiotic prescribing remains a meaningful contributor. OtoNexus is focused on addressing one upstream driver—diagnostic ambiguity at the point of care.
For more information, please explore our investor information page or complete the form below to contact us directly.
Request Information
Call Us
You can also call us directly at (206) 330-0610.
OtoNexus Medical Technologies is more than just a promising investment; it’s an opportunity to be part of a groundbreaking innovation in medical diagnostics with global implications. Our medical device company is based just outside of Seattle, Washington.
Industry Awards and Recognition
OtoNexus Medical Technologies is honored to have received awards and recognition from Keiretsu Capital (Most Valued Company), Keiretsu Forum MidAtlantic (Most Valued Company), Keiretsu Forum Northwest (Most Valued Company), Red Herring (Top 100 North America) Angel Capital Association (Most Investable Company), Puget Sound Business Journal (Washington’s 100 Best Workplaces), MedTech Outlook (Top 10 Infection Control Solution Providers), MedTech Outlook (Top Provider 2024), MedTech Strategist (Startups to Watch), Silicon Review (Innovative Companies to Watch) and Women Corporate Directors (Winner Launch Zone). Please see the full details in our website NEWS.
Support and Non-Dilutive Funding
OtoNexus Medical Technologies has received grants and/or support from the following institutions and groups: National Institutes of Health, National Science Foundation, State of Washington, Life Sciences Discovery Fund, Southwest Pediatric Device Consortium, Philadelphia Pediatric Medical Device Consortium, MedTech Innovator, Advanced Medical Technology Association, Future in Review, Consortium for Technology and Innovation in Pediatrics